When you pick up a prescription, you might see a different name on the bottle than what your doctor wrote. Instead of BrandName, it says the chemical name-like amlodipine instead of Norvasc. That’s a generic drug. They’re cheaper, often by 80% or more, and they’re supposed to work the same way. But do they cause more side effects? That’s what a lot of people wonder-and it’s not just a myth.
Are generics really the same as brand-name drugs?
The U.S. Food and Drug Administration (FDA) says yes. To get approved, a generic drug must deliver the same amount of active ingredient into your bloodstream at the same speed as the brand-name version. That’s called bioequivalence. The allowed range? Between 80% and 125% of the brand’s absorption rate. That sounds like a big gap, but for most drugs, it doesn’t matter. Your body can handle that variation without a problem. Take blood pressure meds like lisinopril or cholesterol drugs like simvastatin. Large studies, including one from PLOS Medicine in 2018 tracking over 38 clinical trials, found no difference in heart attacks, strokes, or hospitalizations between patients on generics versus brand-name versions. In fact, for simvastatin, people on the generic version were less likely to stop taking it-suggesting they actually tolerated it better.Why do some people feel worse on generics?
If the science says they’re the same, why do so many people report new side effects after switching? The answer isn’t always in the active ingredient. It’s in the extras. Generic drugs can use different fillers, dyes, or coatings. These are called inactive ingredients. For most people, they’re harmless. But for someone with a rare allergy or sensitivity-say, to lactose or a specific dye-they might feel off. A pharmacist in Birmingham told me about a patient who developed a rash after switching to a generic version of levothyroxine. The brand used a different binder. Once they switched back, the rash disappeared. It wasn’t the thyroid hormone-it was the filler. Then there’s the nocebo effect. That’s the opposite of placebo. If you believe a generic is inferior, your brain can start making you feel worse. A 2012 study showed that people given identical placebo pills labeled as “generic” reported more side effects than those given the same pills labeled as “brand-name.” The pills didn’t change. The label did.What about the scary studies?
You’ve probably heard about the Ohio State University study from 2022 claiming generics made in India caused 54% more severe side effects-like hospitalizations or death-than those made in the U.S. That sounds alarming. But here’s the catch: the study looked at older generics that had been on the market for years. These aren’t new drugs. They’re the same ones that have been sold for decades. The issue wasn’t the country of origin-it was the fact that some manufacturers cut corners over time to stay profitable. The FDA inspects over 1,700 generic manufacturing sites worldwide. More than 60% are outside the U.S., mostly in India and China. In 2022, 12% of foreign facilities got flagged for serious quality issues. That’s a red flag. But it doesn’t mean every Indian-made generic is unsafe. It means you need to trust the regulator, not the label. The FDA doesn’t care where a drug is made-it cares if it meets their standards. And they’ve ramped up inspections: from 42 in 2010 to 317 in 2022.
Which drugs are riskier to switch?
Not all drugs are created equal. Some have what’s called a narrow therapeutic index-meaning the difference between a helpful dose and a harmful one is tiny. For these, even small changes in absorption can matter. - Levothyroxine (for thyroid): Even a 5% change in absorption can throw your hormone levels off. Many endocrinologists recommend sticking with the same brand or generic manufacturer. - Warfarin (blood thinner): Patients often report unstable INR levels after switching between generics. The FDA’s adverse event database shows a pattern here. - Phenytoin (seizure control): Small variations can trigger seizures. - Bupropion (for depression and smoking): Many patients report increased anxiety or insomnia after switching to generic versions, even though bioequivalence tests say they’re the same. If you’re on one of these, talk to your doctor. Ask them to write “Dispense as Written” on your prescription. It might cost more, but it keeps your treatment consistent.What do real patients say?
Online forums are full of stories. On Reddit’s r/pharmacy, users report everything from brain fog to heart palpitations after switching. One user wrote: “I was stable on Synthroid for 10 years. Switched to generic. My TSH went from 1.8 to 7.2 in two months. I felt like I was drowning.” A Consumer Reports survey in 2022 found 28% of 2,145 respondents said they experienced different side effects after switching to a generic. But here’s what they didn’t measure: were they also stressed about the switch? Did they read articles about generics being dangerous? Did their pharmacist say, “This is cheaper, but it’s not the same”? Those things matter.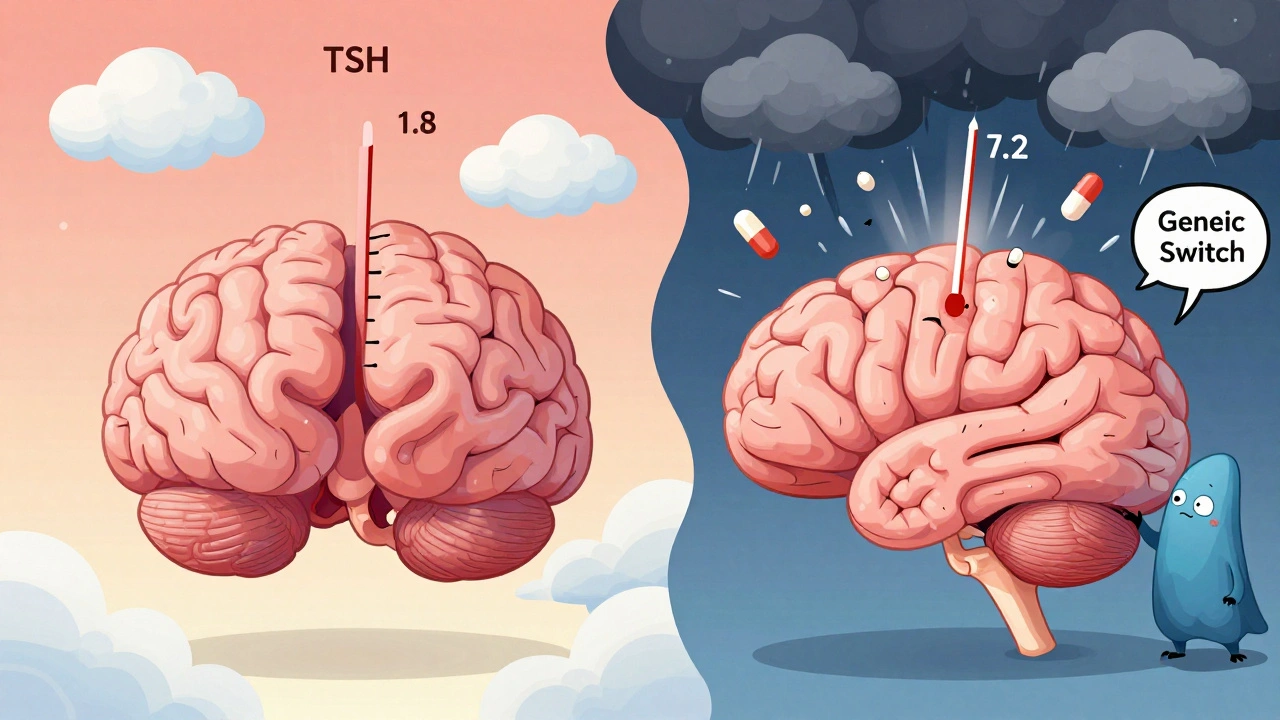
What’s the real risk?
For the vast majority of people, generics are safe and effective. Over 90% of prescriptions in the U.S. are for generics. That’s 8.8 billion pills a year. If they were causing widespread harm, we’d see it in the data. The FDA’s Sentinel Initiative tracks 300 million patient records. Their latest analysis found no spike in emergency room visits for most generic drugs after they hit the market. The only exceptions were a few drugs like losartan and valsartan, where ER visits went up slightly after generics became available. But even then, the study couldn’t prove the switch caused it. Maybe people were more likely to see a doctor after switching because they were worried.What should you do?
If you’re taking a generic drug and feel fine-stick with it. No need to switch back unless you have a reason. If you start feeling worse after switching:- Track your symptoms: When did they start? What changed?
- Check the pill: Look at the imprint code. If it’s different from before, you might have switched manufacturers.
- Talk to your pharmacist: Ask if the generic changed. They can often tell you the manufacturer.
- Ask your doctor: Can we go back to the brand? Or stick with one generic maker?
Bottom line
Generics aren’t riskier for most people. The science supports that. But they’re not always identical. The difference isn’t in the active ingredient-it’s in the extras, the manufacturing, and your expectations. For most drugs, the savings far outweigh the tiny risk. For a few, consistency matters more than cost. Know your drug. Know your body. And don’t let fear make you stop taking medicine that keeps you alive.Are generic drugs less effective than brand-name drugs?
No, generic drugs are required by the FDA to be just as effective as their brand-name counterparts. They must deliver the same active ingredient in the same amount and at the same rate. Large studies involving thousands of patients have found no meaningful difference in outcomes for most conditions, including high blood pressure, cholesterol, and depression. The only exceptions are drugs with a narrow therapeutic index, where even small changes in absorption can matter.
Why do some people have side effects after switching to generics?
Side effects after switching are often not caused by the active ingredient. They can come from differences in inactive ingredients-like fillers, dyes, or coatings-that some people are sensitive to. Psychological factors also play a role: if you believe generics are inferior, you may notice or even feel symptoms more strongly. This is called the nocebo effect. In one study, people given identical placebo pills labeled as "generic" reported more side effects than those given the same pills labeled as "brand-name."
Are generics made in India or China less safe?
The country of manufacture doesn’t automatically mean lower quality. The FDA inspects all facilities, whether in the U.S., India, or China, using the same standards. About 63% of generic drug manufacturing happens outside the U.S., with 32% in India and 18% in China. Some studies have linked older generics from certain manufacturers to higher rates of severe side effects, but that’s tied to specific companies and time periods-not entire countries. The FDA has increased inspections dramatically since 2010, and most facilities now meet safety standards.
Which medications should I avoid switching to generics?
Avoid switching generics for drugs with a narrow therapeutic index, where small changes in blood levels can cause serious problems. These include levothyroxine (for thyroid), warfarin (blood thinner), phenytoin (for seizures), and some antidepressants like bupropion. For these, your doctor may recommend sticking with one brand or manufacturer. Always ask your doctor or pharmacist if your drug falls into this category.
Can I ask my pharmacist to give me the same generic each time?
Yes. You can ask your pharmacist to fill your prescription with the same generic manufacturer each time. Some pharmacies will do this automatically if you ask. You can also ask your doctor to write "Dispense as Written" on your prescription, which legally prevents the pharmacy from switching manufacturers without your consent. This is especially helpful for high-risk medications.
How do I know if my generic has changed?
Check the pill itself. Generic pills have an imprint code-a letter or number stamped on them. If it looks different from your last refill, the manufacturer changed. You can also ask your pharmacist: "Which company made this batch?" Most will tell you. If you notice new side effects after a change, report it to your doctor and consider switching back.
Is it safe to save money by using generics?
Yes, for most people, generics are not only safe but smart. They save patients and the healthcare system billions each year. Over 90% of prescriptions in the U.S. are filled with generics, and the vast majority of users experience no issues. The benefits of affordability and access far outweigh the minimal risks for most medications. Only in rare cases-like with drugs that have a narrow therapeutic index-should cost be weighed against consistency.






Haley P Law
December 8, 2025 AT 09:28Andrea DeWinter
December 9, 2025 AT 19:12George Taylor
December 11, 2025 AT 03:43ian septian
December 11, 2025 AT 16:24Chris Marel
December 12, 2025 AT 23:20Evelyn Pastrana
December 14, 2025 AT 11:54Nikhil Pattni
December 14, 2025 AT 20:10Arun Kumar Raut
December 15, 2025 AT 12:24precious amzy
December 16, 2025 AT 08:44Carina M
December 17, 2025 AT 21:23William Umstattd
December 18, 2025 AT 11:30Elliot Barrett
December 19, 2025 AT 19:23Tejas Bubane
December 20, 2025 AT 04:43