Diuretic Electrolyte & Interaction Checker
How it works
Select your diuretic class and potential interacting medications to see expected electrolyte changes and clinical warnings.
When you hear the word diuretics are a group of medicines that boost urine output by blocking sodium reabsorption in the kidney, you might picture a simple “water pill”. In reality, the story is far richer: each class reshapes the body’s salt balance, can trigger life‑threatening electrolyte swings, and plays a risky game with countless other drugs. This guide walks you through the most common electrolyte changes, the strongest drug‑pair warnings, and a handful of practical tips to keep patients safe.
Why Different Diuretic Classes Matter
All diuretics share a core idea - stop the kidney from re‑absorbing sodium, and water follows. Where they differ is the nephron segment they target:
- Loop diuretics (e.g., furosemide, bumetanide) hit the thick ascending limb’s Na⁺‑K⁺‑2Cl⁻ (NKCC2) transporter, responsible for about 25% of filtered sodium.
- Thiazide diuretics (e.g., hydrochlorothiazide, chlorthalidone) block the Na⁺‑Cl⁻ channel in the distal convoluted tubule, handling roughly 5‑7% of sodium.
- Potassium‑sparing diuretics (e.g., spironolactone, amiloride) either antagonise aldosterone receptors or inhibit the epithelial sodium channel (ENaC) in the collecting duct, causing minimal sodium loss but preserving potassium.
Because the amount of sodium flushed varies, the resulting electrolyte picture also shifts dramatically.
Electrolyte Shifts by Class
Large real‑world studies give us clear odds ratios for each electrolyte problem. Below is a quick snapshot of what clinicians see most often.
| Diuretic class | Typical sodium effect | Typical potassium effect | Key clinical warning |
|---|---|---|---|
| Loop | ↑ risk of hypernatremia (OR 1.87) | ↓ risk of hypokalemia (OR 2.31) | Monitor urine output; avoid high‑dose NSAIDs |
| Thiazide | ↑ risk of hyponatremia (OR 3.15) | ↓ risk of hypokalemia (OR 1.94) | Start low in elderly; watch for dehydration |
| Potassium‑sparing | ↑ risk of hyponatremia (OR 1.78) | ↑ risk of hyperkalemia (OR 4.23) | Check serum K⁺ within 3‑7 days, especially with ACE‑I/ARB |
Even modest shifts matter. A 2023 meta‑analysis showed that mild hyponatremia lifts in‑hospital mortality by 1.45 ×, while severe hyperkalemia pushes it up to 2.87 ×. The takeaway? Small lab changes can have big outcomes, and you need a monitoring plan that matches the drug’s potency.
Top Drug Interactions You Can’t Ignore
Diuretics rarely act alone. Here are the most frequent pairings that flip the safety curve.
- NSAIDs (e.g., ibuprofen, indomethacin): Cut renal prostaglandins, dropping loop diuretic effectiveness by up to 50 %. The result is fluid retention despite high doses.
- ACE inhibitors / ARBs: Reduce aldosterone‑driven potassium loss, which is great for thiazides but dangerous with potassium‑sparing agents. Combined use can lift serum potassium by more than 1 mmol/L.
- SGLT2 inhibitors (dapagliflozin, empagliflozin): Lower proximal sodium reabsorption, sending extra load downstream where loop diuretics act. A 2020 study recorded a 36 % boost in bumetanide’s natriuresis when paired with dapagliflozin.
- Trimethoprim‑sulfamethoxazole: Blocks renal tubular potassium secretion, provoking severe hyperkalemia in patients already on spironolactone. Case reports describe K⁺ spikes over 6.5 mmol/L within days.
- High‑dose thiazides + loop diuretics: Intended for diuretic‑resistant edema, but can precipitate acute kidney injury in 22 % of cases and severe hyponatremia in 15 % (DOSE trial).
When you add any of these agents, plan a tighter lab schedule: check electrolytes within 48 hours, then every 3‑5 days until you’re sure the combination is stable.
Managing Diuretic Resistance
Resistance shows up when the body compensates - usually via up‑regulation of downstream sodium transporters. A classic pattern: after 3 days of high‑dose furosemide, distal tubule sodium reabsorption can jump 40 %. The practical response is “sequential nephron blockade.”
- Start with a loop diuretic (adjust dose to eGFR). For eGFR < 30 mL/min, use 1.5 mg kg⁻¹ furosemide.
- Add a thiazide (e.g., metolazone 5‑10 mg) if urine output stalls.
- If potassium drops below 3.5 mmol/L, consider a potassium‑sparing agent or give oral potassium chloride.
Crucial check‑points: fractional excretion of sodium (FeNa) < 1 % signals poor response, while a FeNa > 2 % after adding a thiazide usually means the combo is working.
Practical Monitoring Blueprint
Every clinician needs a simple lab schedule. The 2023 UpToDate guidance suggests:
- Baseline electrolytes before the first dose.
- Re‑check at 3‑7 days for new prescriptions or dose changes.
- Monthly labs for stable chronic therapy.
- Every 24‑48 hours during hospital‑based uptitration or when combining three agents.
For loop diuretics, remember the peak effect arrives in ~30 minutes IV and 1‑2 hours orally, lasting 6‑8 hours. Thiazides peak later (2‑4 hours) but linger 12‑24 hours, which explains why they’re handy for once‑daily hypertension control.
Emerging Trends: Smart Combinations and AI‑Driven Dosing
Two big shifts are reshaping practice:
- SGLT2 inhibitor “diuretic boosters” - 2023 ACC/AHA guidelines now list dapagliflozin alongside loop diuretics for heart‑failure patients, cutting overall loop doses by ~28 %.
- AI dosing platforms - Pilot work at Mayo Clinic shows algorithm‑driven adjustments cut electrolyte emergencies by 40 % compared with standard nurse‑driven protocols.
Both trends emphasize precision: use biomarkers like urinary aldosterone or fractional excretion of chloride to decide which add‑on will give the most natriuresis with the fewest lab swings.
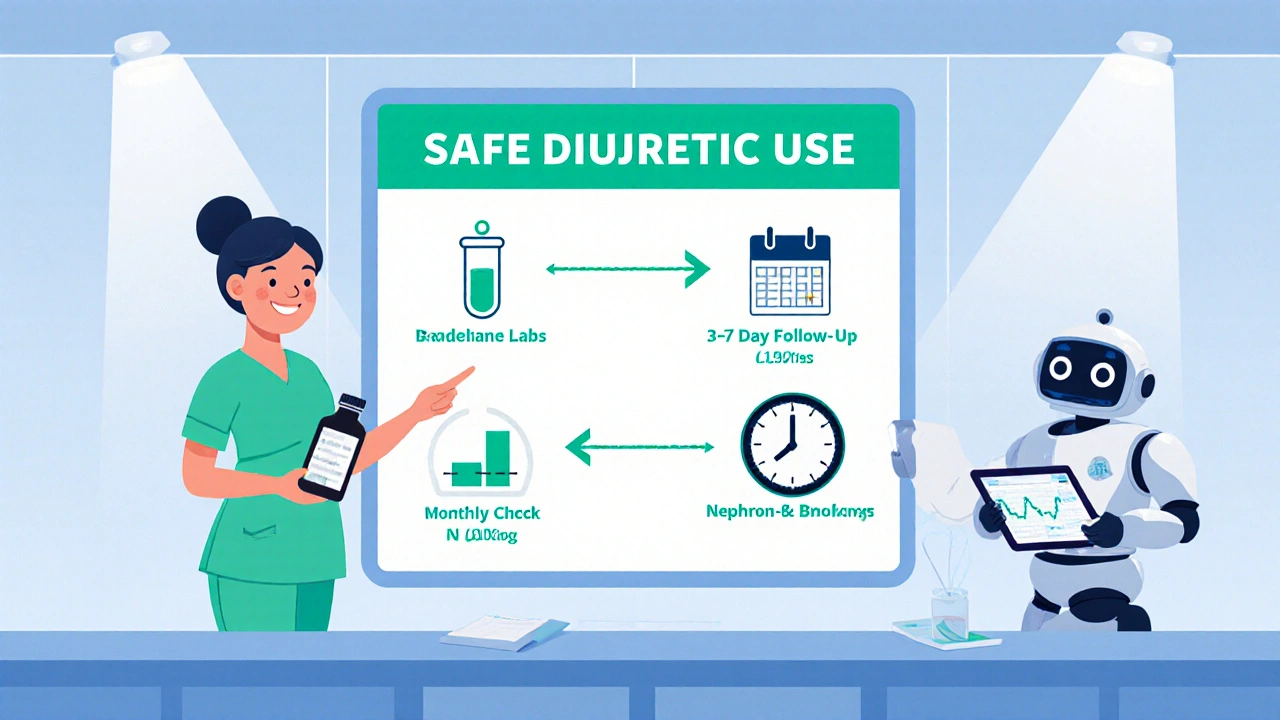
Quick Checklist for Safe Diuretic Use
- Identify the primary diuretic class and match it to renal function.
- Screen for interacting drugs: NSAIDs, ACE‑I/ARB, SGLT2‑i, trimethoprim‑sulfa.
- Order baseline electrolytes (Na⁺, K⁺, Mg²⁺, Cl⁻) and creatinine.
- Re‑check labs within 3‑7 days, then monthly if stable.
- Watch for symptoms: confusion (hyponatremia), muscle cramps (hypokalemia), arrhythmia (hyperkalemia).
- If resistance appears, add a second class and adjust monitoring frequency.
Frequently Asked Questions
Why do loop diuretics cause hypernatremia while thiazides cause hyponatremia?
Loop diuretics dump a lot of water but also excrete free water less efficiently, so the remaining fluid becomes relatively sodium‑rich. Thiazides, on the other hand, impair the kidney’s ability to dilute urine in the distal tubule, so excess water stays while sodium gets diluted, leading to low serum sodium.
Can I safely take a potassium‑sparing diuretic with an ACE inhibitor?
It’s possible, but you must monitor potassium closely. The combination can raise serum K⁺ by more than 1 mmol/L, pushing some patients into hyperkalemia. Check labs within a week of starting, then weekly for the first month.
What’s the best way to prevent diuretic‑induced AKI?
Avoid rapid high‑dose combos, keep patients euvolemic, and use urine output monitoring. If you need to combine a loop with a thiazide, start the thiazide at the lowest dose (5 mg) and watch creatinine every 48 hours.
How do SGLT2 inhibitors enhance loop diuretic effect?
SGLT2 inhibitors block glucose‑linked sodium reabsorption in the proximal tubule, sending more sodium downstream to the loop of Henle where loop diuretics act. The extra delivery amplifies natriuresis without extra loop dosing.
When should I switch from a thiazide to a loop diuretic?
If the patient’s eGFR falls below 30 mL/min, thiazides lose effectiveness. Also, if hyponatremia persists despite dose reduction, a loop may be safer because it works higher up the nephron.
Understanding the electrolyte dance and the drug‑interaction maze lets you keep patients comfortable, avoid hospital readmissions, and use diuretics exactly where they shine. Stay vigilant, check labs, and remember that a small tweak in dosing or an added medication can make the difference between a smooth fluid balance and a dangerous electrolyte storm.





eko lennon
October 25, 2025 AT 19:37Stepping into the realm of diuretics is like watching a high‑stakes drama unfold in the kidneys, where each class takes center stage and the electrolytes become the tragic heroes.
First, the loop diuretics rush onto the scene, dumping massive amounts of sodium and water, only to leave a stubborn surplus of sodium that can push serum sodium sky‑high, a scenario that feels like the climax of a thriller.
Meanwhile, thiazides slip in with a quieter but equally insidious plot, nudging the distal tubule to retain water while shedding sodium, which often culminates in a bewildering hyponatremia that catches clinicians off‑guard.
The potassium‑sparing agents then enter like the misunderstood anti‑hero, shielding potassium from loss but sometimes over‑protecting it, leading to hyperkalemia that can turn a routine visit into a life‑threatening emergency.
What makes this saga even more complex is the cast of drug interactions that loom like villains lurking in the shadows, ready to sabotage any carefully crafted regimen.
NSAIDs, for example, act as silent saboteurs, blunting the prostaglandin‑mediated response that loop diuretics rely on, effectively muting their diuretic punch by up to half.
When an ACE inhibitor or ARB partners with a potassium‑sparing diuretic, the duo can amplify potassium retention to dangerous levels, a plot twist that often requires urgent lab checks.
Adding an SGLT2 inhibitor is like giving the main character a secret weapon, boosting natriuresis downstream and allowing a reduction in loop dose, but only if the script is rewritten with vigilant monitoring.
Trimethoprim‑sulfamethoxazole, on the other hand, can masquerade as an innocent companion while secretly blocking renal potassium excretion, setting the stage for sudden spikes in serum potassium.
Even the most well‑intentioned combination of a high‑dose thiazide with a loop can backfire, precipitating acute kidney injury and severe hyponatremia in a considerable fraction of patients, an outcome no storyteller wants.
To navigate this labyrinth, clinicians must adopt a systematic monitoring blueprint: baseline electrolytes, repeat labs within three to seven days of any change, and more frequent checks when multiple agents are combined.
Understanding the fractional excretion of sodium helps differentiate true diuretic resistance from mere under‑dosing, guiding the addition of a second class in a sequential nephron blockade strategy.
When resistance does appear, the classic “loop‑then‑thiazide‑then‑potassium‑saver” algorithm can resurrect urine output, but only if potassium levels are vigilantly guarded.
The emerging wave of AI‑driven dosing platforms promises to cut electrolyte emergencies by a staggering forty percent, reminding us that technology can be the unsung hero in this narrative.
Yet, even the smartest algorithm cannot replace the bedside intuition that prompts a nurse to check a patient's weight after a new loop is started.
In the end, the drama of diuretics teaches us that every dose adjustment, every drug pair, and every lab value is a line in a script where the patient’s safety is the ultimate climax.
Sunita Basnet
October 25, 2025 AT 23:47Diuretics are the linchpin of natriuresis and mastering their pharmacodynamics can transform volume management.
By targeting distinct nephron segments you can fine‑tune osmotic gradients and avoid iatrogenic dysnatremia.
Remember loop diuretics drive robust diuresis thiazides offer steady outpatient control and potassium‑sparing agents safeguard serum K⁺.
Combine wisely and your patients will stay euvolemic and protected from adverse electrolyte swings.
Diane Holding
October 26, 2025 AT 02:57Great overview; just a heads‑up that FeNa >2 % after adding a thiazide usually signals effective blockade.
Manish Verma
October 26, 2025 AT 07:07Honestly, you’re glossing over the fact that in many low‑resource settings NSAIDs are freely over‑the‑counter, turning any loop regimen into a flop – the data is clear, you can’t trust a “friendly” drug combo without strict monitoring.
Lionel du Plessis
October 26, 2025 AT 11:17Loops knock out 25 % of filtered Na⁺ and give a brisk diuresis but they also risk hypernatremia if the patient isn’t water‑replete.
Thiazides shave off 5‑7 % of Na⁺ and are great for chronic hypertension yet they love to cause hyponatremia in the elderly.
Potassium‑sparing agents keep K⁺ levels up but watch out for ACE‑I synergy‑induced hyperkalaemia.
Overall the key is to match the nephron target to the patient’s renal function and comorbidities.
Andrae Powel
October 26, 2025 AT 15:27I’ve seen patients on spironolactone develop sudden K⁺ spikes after a course of trimethoprim, so I always order a potassium panel within 48 hours of starting the antibiotic.
It also helps to educate them on low‑potassium foods and the signs of arrhythmia, which can make a big difference in preventing an emergency.
Leanne Henderson
October 26, 2025 AT 19:37Wow, this guide really nails it, you’ve covered everything from the basics to the cutting‑edge AI dosing tools, and I love how you’ve broken down the monitoring schedule into bite‑size steps, making it super easy to follow, especially for busy clinicians!
Edward Brown
October 26, 2025 AT 23:47They hide the real risks behind fancy charts